Atom Thomson
Learning Objectives
- Kelemahan Teori Atom Thomson
- Kelebihan Dan Kekurangan Atom Thomson
- Teori Atom Thomson
- Model Atom Thomson
- Explain what a model is.
- Describe the “plum pudding” model of the atom.
Thomson’s model was known as the 'Plum Pudding Model” (or 'Raisin Bread Model.' ) As each atom was a sphere filled with a positively charged fluid, known as the “pudding”. Scattered in this fluid were negatively charged electrons, these were the “plums” in the pudding. In 1904 Thomson suggested a model of the atom as a sphere of positive matter in which electrons are positioned by electrostatic forces. His efforts to estimate the number of electrons in an atom from measurements of the scattering of light, X, beta, and gamma rays initiated the research trajectory along which his student Ernest Rutherford moved. √Atom JJ Thomson Pengertian Atom. Atom adalah sebuah satuan dasar materi, yang terdiri dari (inti atom) serta awan elektron yag bermuatan. Teori Atom Thomson. Model teori atom Thomson muncul setelah dikemukakannya sebuah teori Dalton pada tahun. The Intel Atom processor and 4GB of RAM allow smooth multitasking, while the 4000 mAh lithium-ion battery offers up to seven hours of use. Mail merge toolkit cracked version download. This Thomson Neo laptop has a 10.1-inch WSVGA display and Intel HD 400 integrated graphics for clear visuals, and the 64GB of eMMC storage provide fast startups.
What is this model airplane composed of?
Millions of children over the years have enjoyed building models—this model airplane is one example of the types of models that can be constructed. Perhaps sixty years ago the models were made of balsa wood, a very light material. Parts would be cut by hand, carefully glued together, and then covered with paper or other fabric.
The development of plastics made the construction of model aircraft much simpler in many respects. And, the end-product is more durable and damage-proof.
A model serves a useful purpose—it gives us an idea of what the real thing is like. The model plane seen above has wings, a tail, and an engine just like the real thing. This model also has a propeller, as is the case with most small planes and some smaller passenger planes. However, the model is not the real thing. We certainly cannot fly people or cargo in the model (besides maybe a tiny mouse), but we can get some idea of what a real plane looks like and how it works.
Science uses many models to explain ideas. We model the electron as a very small particle with a negative charge. That gives us a picture, but a very incomplete one. This picture works fine for most chemists, but is inadequate for a physicist. Models give us a start toward understanding structures and processes, but certainly are not a complete representation of the entity we are examining.
Atomic Models
The electron was discovered by J.J. Thomson in 1897. The existence of protons was also known, as was the fact that atoms were neutral in charge. Since the intact atom had no net charge and the electron and proton had opposite charges, the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom. This is a difficult task because of the incredibly small size of the atom. Therefore, scientists set out to design a model of what they believed the atom could look like. The goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.
Following the discovery of the electron, J.J. Thomson developed what became known as the “plum pudding” model in 1904. Plum pudding is an English dessert similar to a blueberry muffin. In Thomson’s plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge like blueberries stuck into a muffin. The positive matter was thought to be jelly- like or a thick soup. The electrons were somewhat mobile. As they got closer to the outer portion of the atom, the positive charge in the region was greater than the neighboring negative charges and the electron would be pulled back more toward the center region of the atom.
However, this model of the atom soon gave way to a new model developed by New Zealander Ernest Rutherford (1871-1937) about five years later. Thomson did still receive many honors during his lifetime, including being awarded the Nobel Prize in Physics in 1906 and a knighthood in 1908.
Summary
- A model gives an idea of what something looks like, but is not the real thing.
- The “plum pudding” model of the atom consisted of a uniform sphere of positive charge with negative electrons embedded in the sphere.

Practice
Use the link below to answer the following questions:
- In the plum pudding model of the atom, what are the plums?
- In this model, what is the dough?
- What was the major purpose of the plum pudding model?
- How is this model different from modern modes of the atom?
Review
- What is a model?
- Why are models useful in science?
- In Thomson’s model of the atom, where were the electrons?
- What was the positive charge in this model?
- What kept the electrons in the atom?
- Whose model replaced Thomson’s?
- What awards did Thomson receive?
Glossary
- atomic model: When scientists set out to design a model of what they believed the atom could look like, the goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.
- plum pudding : In 1904 J.J. Thomson developed this model. The electrons were stuck into a uniform lump of positive charge like blueberries in a muffin. The positive matter was thought to be jelly- like or a thick soup. The electrons could move around somewhat. As they got closer to the outer portion of the atom, the positive charge in the region was greater than the neighboring negative charges and the electron would be pulled back more toward the center region of the atom.
References
- User:Fokker/Wikimedia Commons. http://commons.wikimedia.org/wiki/File:3dx-I.JPG.
- User:Fastfission/Wikimedia Commons. http://commons.wikimedia.org/wiki/File:Plum_pudding_atom.svg.
Main Difference – Thomson vs Rutherford Model of Atom
Thomson model of atom is one of the earliest models to describe the structure of atoms. This model is also known as the plum pudding model due to its resemblance to a plum pudding. This explains that this atom is a spherical structure made out of a positively charged solid material and the electrons are embedded in that solid. But this model was rejected after the discovery of atomic nucleus. Rutherford model of atom describes the atomic nucleus and the location of electrons in an atom. It was proposed who described that an atom is composed of a central solid core which is positively charged and electrons are located surrounding this solid core. However, this model was also rejected because it could not explain why the electrons are not attracted to the nucleus. The main difference between Thomson and Rutherford model of atom is that Thomson model does not give details about the atomic nucleus whereas Rutherford model explains about the nucleus.
Key Areas Covered
1. What is Thomson Model of Atom
– Definition, Model, Drawbacks
2. What is Rutherford Model of Atom
– Definition, Model, Drawbacks
3. What is the Difference Between Thomson and Rutherford Model of Atom
– Comparison of Key Differences
Key Terms: Alpha Particles, Atom, Electron, Gold Foil Experiment, Nucleus, Plum Pudding Model, Rutherford Model of Atom, Thomson Model of Atom
What is Thomson Model of Atom
Thomson model of atom is the structure of an atom proposed by the scientist, J.J.Thomson, who was the first person to discover the electron. Soon after the discovery of the electron, the atomic model was proposed saying that the structure of an atom is like a “plum pudding”.
Thomson model of atom is described base on three main facts:
- Atoms are composed of electrons.
- Electrons are negatively charged particles.
- Atoms are neutrally charged.
Thomson proposed that since electrons are negatively charged and atoms are neutrally charged, there should be a positive charge in the atom in order to neutralize the negative charge of electrons. He proposed that the atom is a solid, positively charged, spherical structure and electrons are embedded in that sphere. Since this structure looks like a pudding with plums scattered on it, it was called the plum pudding structure of atom. However, this structure was rejected after the discovery of atomic nucleus.
Drawbacks of Thomson Model of Atom
- No details about atomic nucleus.
- No details about atomic orbitals.
- No explanation about the protons or neutrons.
- States that the atom has a uniform distribution of mass, which is wrong.
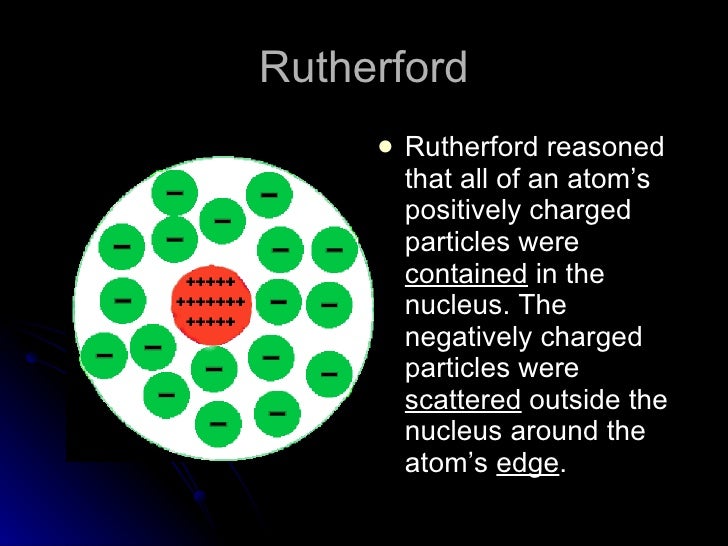
What is Rutherford Model of Atom
Rutherford model of atom describes that the atom is composed of an atomic nucleus and electrons surrounding the nucleus. This model caused to reject the Thomson model of atom. Rutherford model was proposed by Ernst Rutherford after the discovery of the atomic nucleus.
The gold foil experiment was used by Rutherford to propose this atomic model. In this experiment, alpha particles were bombarded through a gold foil; they were expected to go straight through the gold foil. But instead of straight penetration, alpha particles turned into different directions. This experiment indicated that there is a positively charged, solid material in the middle of the atom while the rest of the atom has more empty space. This solid core was named as the nucleus.
According to this model,
- The atom is composed of a positively charged center which is called the nucleus. This center contained the mass of the atom.
- Electrons are located outside the nucleus in orbitals at a considerable distance.
- The number of electrons is equal to the number of positive charges (later called protons) in the nucleus.
- The volume of the nucleus is negligible when compared to the volume of the atom. Hence, most of the space in the atom is empty.
Drawbacks of Rutherford Model of Atom
Later, Rutherford model was also rejected because it could not explain why the positively charged nucleus and electrons are not attracted to each other.
Difference Between Thomson and Rutherford Model of Atom
Definition
Thomson Model of Atom: Thomson model of atom states that electrons are embedded in a positively charged solid material which is spherical in shape.
Rutherford Model of Atom: Rutherford model of atom describes that an atom is composed of an atomic nucleus and electrons surrounding the nucleus.
Nucleus
Thomson Model of Atom: Thomson model of atom does not give any details about the atomic nucleus.
Rutherford Model of Atom:Rutherford model of atom explains about the atomic nucleus.
Electrons
Huawei mobile partner mac catalina. Thomson Model of Atom: Thomson model of atom states that electrons are embedded in a solid material.
Rutherford Model of Atom:Rutherford model of atom states that electrons are located around a central solid material.
Shape of Atom
Thomson Model of Atom: Thomson model of atom indicates that the atom is a spherical structure.
Kelemahan Teori Atom Thomson
Rutherford Model of Atom:Rutherford model of atom indicates that an atom has a central solid core surrounded by electrons.
Conclusion
Thomson model of atom and Rutherford model of atom are two models proposed by J.J.Thomson and Ernest Rutherford, respectively in order to explain the structure of an atom. The main difference between Thomson and Rutherford model of atom is that Thomson model does not give details about the atomic nucleus whereas Rutherford model explains about the nucleus.
Kelebihan Dan Kekurangan Atom Thomson
References:
Teori Atom Thomson
1. “Thomson Atomic Model & its Limitations | Development Of Atomic Model.” Chemistry, Byjus Classes, 7 Nov. 2017, Available here.
2. “Thomson atomic model.” Encyclopædia Britannica, Encyclopædia Britannica, inc., 27 Dec. 2013, Available here.
3. “Geiger–Marsden experiment.” Wikipedia, Wikimedia Foundation, 8 Nov. 2017, Available here.
Image Courtesy:

Model Atom Thomson
1. “PlumPuddingModel ManyCorpuscles” By Tjlafave – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Rutherfordsches Atommodell” (Public Domain) via Commons Wikimedia
