The Mass Number Of An Atom Is The Number Of
Contents
- Atomic number and Mass number
- Isotopes
Atom
An atom is the smallest particle of an element which can take part in chemical reaction. Atom consists of three fundamental particles i.e. proton, neutron and electron. Atoms of same elements are similar in properties whereas atoms of different elements are different in properties. Example:- ‘H’ represent the atom of hydrogen.
Proton is positively charged and electron is negatively charged particle. In an atom, number of protons = number of electrons. Hence, the net charge present in an atom is zero i.e. a free atom is chargeless.
An atom of an element with an atomic number 48 and mass number 120 contains. 48 protons, 48 electrons, and 72 neutrons. How do the isotopes hydrogen-2 and hydrogen-3 differ? Hydrogen-3 has two neutrons. The number 80 in the name bromine-80 represents. A mass number is the total number of protons and neutrons found in an atom.To find the mass number, you must know the number of neutrons in that particular isotope of the element and then add the. Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number (represented by the letter A) is defined as the total number of protons and neutrons in an atom.
Atomic number and Mass number
Atomic number :
- Atomic number is the number of protons present in an atom.
- The modern periodic table is arranged in order of increasing atomic number.

Mass number and Atomic mass :
- Mass number is the sum of the number of protons and the number of neutrons present in an atom. It is a whole number.
Mass no. of an atom = No. of protons + No. of neutrons
- Atomic mass is the average mass of the all of the isotopes of that element. It is a decimal number.
- For example: Hydrogen has three isotopes – 1H1, 1H2 and 1H3 having mass number 1, 2 and 3 respectively. Naturally occurring hydrogen contains about 99.985% of protium, 0.014% of deuterium and 0.001 % of tritium. Therefore the atomic mass of hydrogen is 1.00784 amu.
- The atomic mass of an element element is measured in atomic mass unit (amu, also known as Daltons ‘ D’or unified atomic mass unit ‘u’).
- 1amu = 1.66 x 10-24 grams. 1gm = 6.022 x 1023 amu ( i.e. Avogadro’s number).
Here,
- Atomic number = Number of protons = Number of electrons = 13
- Mass number = No. of protons + No. of neutrons
- No. of neutrons = Mass number – No. of protons = 27-13 = 14.
Atomic mass of first 20 elements
| Atomic number | Element | Atomic mass |
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
| 5 | Boron | 10.81 |
| 6 | Carbon | 12.011 |
| 7 | Nitrogen | 14.007 |
| 8 | Oxygen | 15.999 |
| 9 | Fluorine | 18.998 |
| 10 | Neon | 20.180 |
| 11 | Sodium | 22.990 |
| 12 | Magnesium | 24.305 |
| 13 | Aluminium | 26.982 |
| 14 | Silicon | 28.085 |
| 15 | Phosphorus | 30.974 |
| 16 | Sulfur | 32.06 |
| 17 | Chlorine | 35.45 |
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
Isotopes
Atoms of the same element having same atomic number but different mass number (atomic mass/weight) are called isotopes. For example:
Isotopes of hydrogen :
There are three isotopes of hydrogen:
- Protium or ordinary hydrogen
- Deuterium or heavy hydrogen
- Tritium or radioactive hydrogen.
| Name | Protium | Deuterium | Tritium |
| Symbol | 1H or H | 2H or D | 3H or T |
| No. of protons(P) | 1 | 1 | 1 |
| No. of neutrons(n) | 0 | 1 | 2 |
| No. of electrons(e) | 1 | 1 | 1 |
| Atomic no.(Z) | 1 | 1 | 1 |
| Mass no.(A) | 1 | 2 | 3 |
Naturally occurring hydrogen contains about 99.985% of protium, 0.014% of deuterium and 0.001 % of tritium.
Isotopes have different physical properties since they differ in their mass number.
They have same chemical properties since their electronic configuration is same. However, they differ in the rate of chemical reaction. For example, D2 reacts with Cl2 about 13 times slower than H2 does. The different in rate of reaction due to difference in mass of the atoms of the same element is called isotope effect.
Some other examples of isotopic elements :
| Elements | Isotopes | Most abundant isotope |
| Carbon | 6C12, 6C13, 6C14 | 6C12 |
| Nitrogen | 7N14, 7N15 | 7N14 |
| Oxygen | 8O16, 8O17, 8O18 | 8O16 |
| Sulphur | 16S32, 16S33, 16S34, 16S36 | 16S32 |
| Chlorine | 17Cl35, 17S37 | 17Cl35 |
Isobars

Atoms of different elements having different atomic number but same mass number are called isobars. For example :
18Ar40, 19K40 and 20Ca40
Isotones
Atoms of different elements having different atomic number and mass number but same number of neutrons are called isotones. For example :
6C14, 7N15 and 8O16
Objective questions and their answers
1. Which of the following is known as heavy hydrogen?
a. Protium c. Tritium
b. Deuterium d. Para hydrogen
2. Which of the following is known as radioactive hydrogen?
a. Protium c. Tritium
b. Deuterium d. Para hydrogen
3. Least abundant isotope of hydrogen is:
a. Protium c. Tritium
b. Deuterium d. Heavy hydrogen
4. Diamond and graphite are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
5. 6C14 and 8O16 are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
6. 6C14 and 7N14 are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
7. All particles residing inside the nucleus of an atom are termed as:
a. Protons c. Electrons
b. Neutrons d. Nucleons
8. What makes the atomic mass fractional ?
a.Prerence of isotopes
b. Number of unpaired electrons
c. Spherical shape
d. Quantum number.
9. Which of the following are not isotopes:
a. 1H1 and 1H3
b. 18K40 and 20Ca40
c. 6C14 and 7N14
d. Both b and c.
10. Charge present in the nucleus of an atom is :
a. Positive c. Chargeless
b. Negative d. Both +Ve and -Ve
11. Molecular weight of heavy water is :
a. 16 c. 20
b. 18 d. 22
Answers :
1. b 2. c 3. c
4. d [Note : different forms of same element having different properties are called allotropes]
5. c 6. b 7. d
8. a 9. d 10. a
Mass Number Is
11. c Note :Heavy water– Deuterium oxide (D2O) is called heavy water. It’s molecular weight is 20 and boiling paint is 101.50C and melting point is 3.80C.
References
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
Learning Objectives
- Define mass number.
- Calculate the mass number when given number of protons and neutrons.
- Calculate number of neutrons when given atomic number.
How can you determine the mass of a chemical?
Often a student will need to weigh out a chemical for an experiment. If he or she uses a watch glass (a small, round piece that will hold the solid chemical), the weight of the watch glass must be determined first. Then the solid is added to the glass and the weight of the glass plus the solid is measured. The balance reading will be the total of the glass plus the chemical.
History of Atomic Weight Determinations
As a part of his research on atoms, John Dalton determined a number of atomic weights of elements in the early 1800s. Atomic weights were the basis for the periodic table that Mendeleev developed. Originally all atomic weights were based on a comparison to hydrogen, which has an atomic weight of one. After the discovery of the proton, scientists assumed that the weight of an atom was essentially that of the protons—electrons were known to contribute almost nothing to the atomic weight of the element.
This approach worked until we learned how to determine the number of protons in an element. We then saw that the atomic weight for an element was often twice the number of protons (or more). The discovery of the neutron provided the missing part of the picture. The atomic mass now known to be the sum of the protons and neutrons in the nucleus.
Mass Number
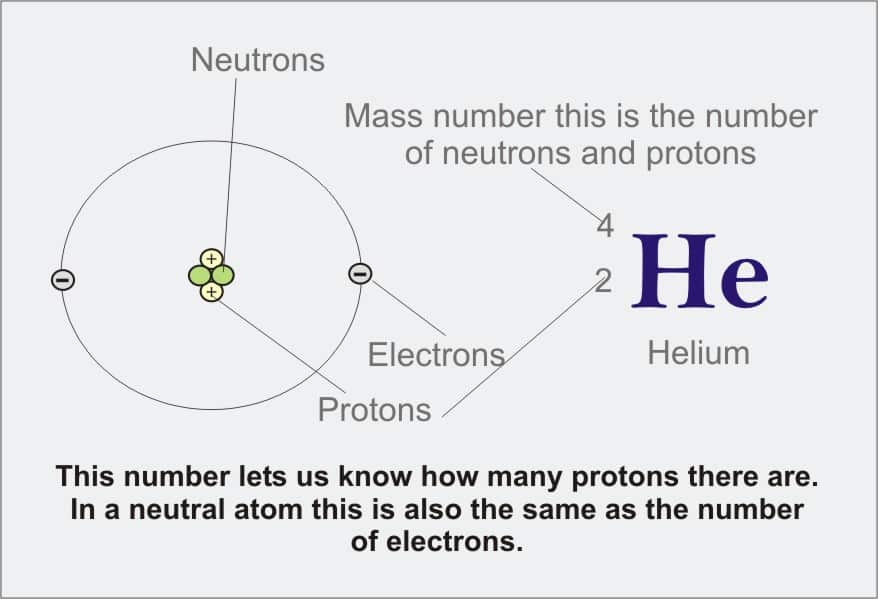
Rutherford showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number is defined as the total number of protons and neutrons in an atom. Consider Table below which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number | Protons | Neutrons | Electrons | Mass Number |
| Hydrogen | H | 1 | 1 | 0 | 1 | 1 |
| Helium | He | 2 | 2 | 2 | 2 | 4 |
| Lithium | Li | 3 | 3 | 4 | 3 | 7 |
| Beryllium | Be | 4 | 4 | 5 | 4 | 9 |
| Boron | B | 5 | 5 | 6 | 5 | 11 |
| Carbon | C | 6 | 6 | 6 | 6 | 12 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since 2 + 2 = 4, we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but further examination of the Table above will show that this is not the case. Lithium, for example has three protons and four neutrons, leaving it with a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
Number of neutrons = mass number − atomic number
Atoms of the element chromium (Cr) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
52 − 24 = 28 neutrons in a chromium atom

The composition of any atom can be illustrated with a shorthand notation using the atomic number and the mass number. Both are written before the chemical symbol, with the mass number written as a superscript and the atomic number written as a subscript. The chromium atom discussed above would be written as [latex]^{52}_{24}text{Cr}[/latex].
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The above atom would be written as chromium-52.
Summary
- The mass number is defined as the total number of protons and neutrons in an atom.
- The number of neutrons = mass number − atomic number.
Practice
Use the link below to answer the following questions:
- What data in the periodic table tells you the number of protons in an atom?
- How do you determine the number of neutrons in an atom?
- What is the mass number for an atom?
Review
- Who first determined atomic weights for elements?
- What were the original atomic weights based on?
- Why were calculations based on numbers of protons not valid for determining atomic weights?
- A tin atom has an atomic number of 50 and a mass number of 118. How many neutrons are present in this atom?
- What is the mass number of a cobalt atom that has 27 protons and 30 neutrons?
The Atomic Mass Of An Element Is
Glossary
- mass number: The total number of protons and neutrons in an atom.
References
- User:Ebultoof/Wikipedia. http://commons.wikimedia.org/wiki/File:Analytical_Balance.JPG.
- D. Mendeleev, Serge Lachinov. http://commons.wikimedia.org/wiki/File:Mendeleev_law.jpg.
